大多数急性肺栓塞来自下肢的深静脉系统,但是它们也来自右心室、盆腔、肾或上肢。 髂股静脉是是最常见的肺栓塞栓子的来源【9-11】。据估计50-80% 髂、股和腘静脉血栓(近端静脉血栓) 来源于腘静脉以下的血栓和向近端蔓延。其它来源于近端静脉内。幸运的是,如果不治疗,多数小腿静脉(calf vein)血栓会自发性溶解,仅有20-30%扩展到近端静脉。多数下肢静脉血栓发展至血流减少的位置,如静脉瓣膜或分叉部位。 当血栓脱落沿血流进入肺,大血栓可以嵌顿到主肺动脉分叉(鞍型肺栓塞),或叶的分支并引起血流动力学的损害。较小的血栓持续进入到末梢分支动脉,最可能导致胸痛,推断由于刺激邻近壁层胸膜的炎性反应。仅有10%导致肺梗死,通常病人同时存在心肺疾病。多数肺栓塞是多发性的,而下叶占大多数【12】。 由于肺栓塞导致的气体交换受损,基于血管床机械性阻塞和不能仅解释为以血管床机械性阻塞和通气与血流灌注比值( ventilation to perfusion ratio)的变化。气体交换异常也与炎性介质的释放相关,导致表面活性物质的释放,肺不张和功能性肺内分流【13】。 低血压由于肺血管阻力( pulmonary vascular resistance ,PVR))增加导致心脏输出减少。PVR的增加来自于血栓导致的肺血管床物理性阻塞和血管收缩,后期由于炎性介质和缺氧的影响。经历肺动脉血栓栓塞的正常人,肺动脉压到血栓大小的相互关系受限于血管收缩等因素。但是当血管床的阻塞到达75%,右心室必须产生收缩压超过50mmHg,平均肺动脉压将近40mmHg 以保持肺动脉灌注【14】。正常的右心室是不能完成这项任务,最终发生心力衰竭。 有心肺疾病历史的患者,心输出比正常人更容易发生实质性恶化。另外,肺栓塞后右心室衰竭在同时存在冠状动脉疾病的患者更常见【15】。 肺栓塞可以分为急性或慢性肺栓塞。急性肺栓塞病人肺血管阻塞后通常即刻出现症状和体征。相反,慢性肺栓塞病人则由于肺动脉高压趋向于数年后逐渐出现的复习困难。
急性肺栓塞可以进一步分为大块肺栓塞(massive)和次大块肺栓塞(submassive)
大块肺栓塞引起低血压,定义为收缩压≤90mmHg,或从基线起收缩压下降≥40mmHg,持续>15分钟。低血压伴有中心静脉压升高或颈静脉怒张,不能解释的心肌梗死,张力性气胸,心包填塞,或新出现心律失常,在任何时候都要怀疑大块肺栓塞【1,2】。这是个灾难性的局面,常导致急性右心室衰竭和死亡。虽然病人有24-72小时危险期,但死亡发生时,通常在1-2小时内【3,4】。PE有时直到尸检之前都不能发现【5,6】。
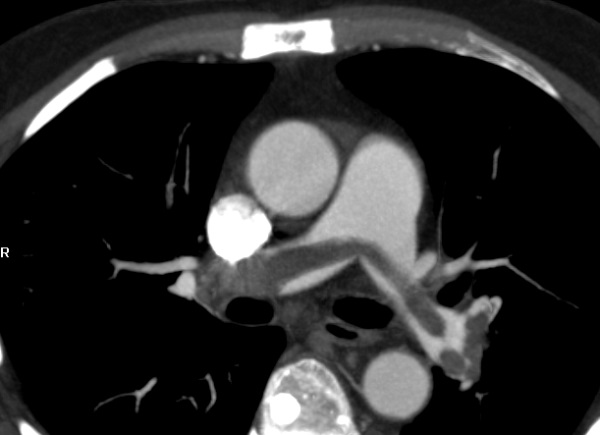
鞍型肺栓塞是血栓卡住(lodge)猪肺动脉分叉进入左右肺动脉。回顾性连续研究546例肺栓塞病人,14例(2.6%)为鞍型肺栓塞,仅有两例为低血压【7】。相似的回顾性研究680例肺栓塞病人,37例(5.4%)有鞍型肺栓塞,其中暂时性低血压发生14%,持续性休克发生8%【8】。因此,鞍型肺栓塞,通常是次大块肺栓塞,和其它类型的肺栓塞一样有相似的治疗反应,死亡率5.4%。
1. Guidelines on diagnosis and management of acute pulmonary embolism. Task Force on Pulmonary Embolism, European Society of Cardiology. Eur Heart J 2000; 21:1301.
2. Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112:e28. 3. COON WW, WILLIS PW. Deep venous thrombosis and pulmonary embolism: prediction, prevention and treatment. Am J Cardiol 1959; 4:611. 4. Soloff LA, Rodman T. Acute pulmonary embolism. II. Clinical. Am Heart J 1967; 74:829.
Bergqvist D, Lindblad B. A 30-year survey of pulmonary embolism verified at autopsy: an analysis of 1274 surgical patients. Br J Surg 1985; 72:105.
5. Goldhaber SZ, Hennekens CH, Evans DA, et al. Factors associated with correct antemortem diagnosis of major pulmonary embolism. Am J Med 1982; 73:822.
6. Goldhaber SZ, Hennekens CH, Evans DA, et al. Factors associated with correct antemortem diagnosis of major pulmonary embolism. Am J Med 1982; 73:822.
7. Ryu JH, Pellikka PA, Froehling DA, et al. Saddle pulmonary embolism diagnosed by CT angiography: frequency, clinical features and outcome. Respir Med 2007; 101:1537.
8. Sardi A, Gluskin J, Guttentag A, et al. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med 2011; 39:2413.
9. Kistner RL, Ball JJ, Nordyke RA, Freeman GC. Incidence of pulmonary embolism in the course of thrombophlebitis of the lower extremities. Am J Surg 1972; 124:169. 10.Moser KM, LeMoine JR. Is embolic risk conditioned by location of deep venous thrombosis? Ann Intern Med 1981; 94:439.
Weinmann EE, Salzman EW. Deep-vein thrombosis. N Engl J Med 1994; 331:1630.
11. van Langevelde K, Srámek A, Vincken PW, et al. Finding the origin of pulmonary emboli with a total-body magnetic resonance direct thrombus imaging technique. Haematologica 2013; 98:309. 12. Moser KM. Venous thromboembolism. Am Rev Respir Dis 1990; 141:235. 13. Nakos G, Kitsiouli EI, Lekka ME. Bronchoalveolar lavage alterations in pulmonary embolism. Am J Respir Crit Care Med 1998; 158:1504. 14. Benotti JR, Dalen JE. The natural history of pulmonary embolism. Clin Chest Med 1984; 5:403. 15. Moser KM, LeMoine JR. Is embolic risk conditioned by location of deep venous thrombosis? Ann Intern Med 1981; 94:439. |

|